Saturday, May 17, 2008
OIL CROSSING BOUNDERIES
light crude oil has traded on 117$ dollars .
wake up> today it is on 127$ dollars a barrel. It is not a demand and supply gape
Dollar is weakening all around the world plus America is beginning to loose so called
war on terror (now every body know the truth)
yesterday bush met the king of Saudi Arabia and appealed him to enhance crude oil production
but it won,t cure the problem
Need of alternative is now greater then ever
Gasoline Diesel Biodiesel Ethanol Cng Bethanol Hydrogen Fuel Cell Alcohal
parson domains
Monday, April 21, 2008
FUEL PRICE ALARMING
during newyork oil trading . the need of alternative fuel (OTHER THEN CRUD OIL PRODUCTS)
is high priority of the whole world
It,s an alarming situation,
not only for poor and developing countries but also for developed and rich countries .
it is the right time for human kind either choose another path (alternative of crud oil specially
renewable) or destruction
did i say DESTRUCTION why? i,ll show you how.
because the O.P.E.C or oil producing countries has refused to enhance oil production giving reasons bla,bla,bla and the people living on this planet don,t want to hear that crap they want cheap energy to live peacefully if they( O,P,E,C ) don,t act then people WILL .
O,P,E,C The Organization of the Petroleum Exporting Countries
there are 12 members (countries) of this organization IRAN, IRAQ, KUWAIT, SAUDI ARABIA
VENEZUELA, QATER, INDONESIA, LIBYA, UAE, ALGERIA , NIGERIA, ECUADOR, & ANGOLA
if i am not wrong the fate of 6 (six) billion people living in 200 countries are totally dependent on the decisions of these countries i think finding a right solution for the oil crisis is much more important then any industrial or bumper crop type revolution these two things can only be achieved if we have sufficient, cheap and ample amount of energy recourses
the fact is that currently we have only few alternative energy sources available most of them are not renewable if does the main problem again ,the price, here is where the story stops
Thursday, April 10, 2008
Liquid Petrolium Gas (LPG),Liquefied Natural Gas (LNG) and Compressed Natural Gas (CNG)
Liquefied Petroleum Gas (LPG), Liquefied Natural Gas (LNG) and Compressed Natural Gas (CNG)
Liquefied Petroleum Gas (LPG), Liquefied Natural Gas (LNG) and Compressed Natural Gas (CNG) are fossil derived fuels and therefore release, one way or another, sequestered greenhouse gases into the atmosphere. As vehicle fuels, they are suitable for use in the two dominant internal combustion engine technologies; spark ignition and compression ignition. Although capable of working in either type of engine there are practical factors which limit their applications to one rather than the other. Broadly speaking LPG is compatible with petrol (gasoline) engines and LNG and CNG with heavy diesel vehicles.Their main claims to fame are that they produce much less tailpipe pollution and can be significantly cheaper per mile to run especially in the UK where users can benefit from government grants, reduced excise duties and other charges. Many vehicles using these gases are dual fuel (aka bi-fuel) and there is a reasonable structure of filling stations, particularly for LPG; as a result they are practical and are 'here and now'.
Liquefied Petroleum Gas is a fuel which can power cars, buses and lorries, however due to factors discussed below, and other alternative fuels being available, LPG is best suited to light vehicles such as cars and small vans which normally run on petrol. Our estimate of energy density* is 65% compared to diesel and about 75% compared to petrol (gasoline).Typically the gas is predominantly propane (C3H8) with some butane (C4H10) derived mainly from oil refineries (also North Sea gas, in the UK). We give the chemical formulae because it is the ratio of carbon to hydrogen which is important; the smaller the ratio of C to H, the better for the environment. It follows that methane (CH4) is a better gas in this respect but only if fully burnt! The gas is liquefied by moderate compression at normal temperatures and is stored in appropriate tanks and cylinders. The liquefaction is necessary to provide a reduction in volume and produce acceptable energy densities. In general this moderate, well tried, process gives it a portability and makes it a fuel with a myriad of applications but the main application discussed here is as a fuel for motor vehicles.LPG vehicles need to be purpose built or they can be converted. It seems that conversions are only practically applicable to petrol vehicles, not diesel because diesel engines need significant modification for this particular gas. Normally they are Bi-fuel which means that they can be run on either LPG or petrol at the flick of a switch, even while motoring. The most notable difference between LPG and petrol or diesel, for cars and vans, is the cost of fuel. As a rough guide, in the UK, the cost per gallon is halved compared to petrol, because the government have reduced the duty by a very substantial amount. In addition to this concession, DETR grants are also available to carry out conversions but they only apply to vehicles less than five years old: grants for cars and light vans were about £700 to £800 in 2004. We also noted in January 2005 that LPG vehicles, eligible for grants, (amongst other low exhaust-polluters) should qualify for 100% exemption from the London Congestion charge. From a local environmental point of view LPG is cleaner than petrol and also diesel, although it is still a fossil fuel and thus its use, as a whole, contributes to global pollution and climate change. At the vehicle exhaust there are less CO, hydrocarbons, nitrous oxides and particulates emitted and it deposits less sulphur in the engine.There are drawbacks to converting vehicles to LPG:
The cost of conversion of a petrol car seems to work out at about £1,100 to £1,500 and it may take a few days to do (a few weeks lead time). According to Andrew Frankel in an article in the Sunday Times (13 Jan. 2002) the estimated cost was greater, from about £1,500 to £2,300. Whichever, the resultant capital outlay dictates that you must do a lot of miles to recoup the cost.
There are relatively few filling stations around the UK, but no doubt more will become available as there are more users (in January 2003 we read that there are well over a thousand stations).
We have also heard of problems caused by non-standard filling nozzles.
The mpg will be less than with conventional fuel (our guess is it will be reduced to about 3/4) but even so fuel costs should be lower, at about 2/3 [ie 1/2 X 4/3 = 2/3].
The range of a tank full of LPG is likely to be less than a tank of petrol, however, with bi-fuel vehicles the total range will be the sum of the two so there is no need to be concerned about finding LPG stations.
The extra volume taken up by the gas tank will reduce the available load space.
There may be a slight performance deficit.
You need to check that the manufacturers guarantee will not be invalidated and we suggest you check whether the insurance premiums will be increased.
If your vehicle uses only leaded petrol the engine may require replacement of the valve seats.
Liquefied Natural Gas fuel (LNG) is produced from a mixture of raw components but is predominantly methane and is compatible with diesel technology, subject to the necessary modifications. Since the composition of methane is CH4, Natural Gas Vehicles (NGVs) are burning fuel with a relatively low carbon to hydrogen ratio. Our estimate of energy density is 60% compared to diesel.LNG cannot be converted to a liquid by pressure alone but must be cooled to a very low temperature (lower than -160°C), a process which removes some impurities such as sulphur and water. The LNG must be stored and transported permanently at around this temperature and this is accomplished by super insulation in a pressurised, double tank system, similar in principle to a thermos flask, together with a venting system to take away vapour. The storage pressure of about 8 bar (8 x atmospheric) is not regarded as very high but because of the insulation requirements the tanks are large, the fuel is only suited to large, heavy diesel vehicles such as trucks, buses and HGVs.Although the energy density is about 60% compared to diesel the fuel costs are much lower and LNG should give lower running costs. Vehicle excise duties and road tax in the UK are reduced for natural gas vehicles and they are exempt from the London Congestion Charge providing they appear on the Powershift register.When compared to diesel, NGVs are quieter and local emissions of pollutants are much reduced but of course the main drawback, global pollution associated with the burning of fossil fuels, still remains.
Compressed Natural Gas (CNG) is, as its name suggests, the close relative of LNG and as a natural gas it has the same basic characteristics. However, because it is not liquefied it has a lower energy density and is stored at very high pressures; about 200 bar. Our estimate of energy density is 25% compared to diesel or 42% compared to LNG.These two factors are a big disadvantage for CNG. Storage and vehicle tanks have to be robust and heavy because of the high pressure requirement. The space taken up on the vehicles by the tanks is significantly more than twice that for LNG tanks (or the range is much less than half) because of the lower energy density.Because it is Natural Gas it attracts financial incentives similar to LNG (details should be checked individually). LNG is much more portable because CNG depots need to be supplied by pipeline and need compressors on site. LNG sites require much less capital investment and are more expensive to run.It certainly seems that CNG is the poor relative of LNG but we can see evidence that it is used for heavy transport in the UK, on a limited number of prescribed highways.
Liquefied Compressed Natural Gas (LCNG). This seems to be a marketing feature so that LNG refuelling stations have the ability to dispense two fuels, LNG and CNG at the same location. LNG can be pressurised and vapourised to give LCNG.
Summary: All of these gases can offer considerable reduction in pollution at the tailpipe, however, since they are fossil fuels in origin their continued use contributes to climate change.Although, in principle, they can be used to replace diesel or petrol, LPG is better suited as a petrol alternative for smaller vehicles such as cars and small vans and LNG and CNG are appropriate for larger diesel vehicles.The fact that conversion of existing engines, using tried and tested methods, is practical (even if not without cost) together with acceptable energy densities makes the use of these fuels an immediate proposition. The fact that the raw supplies are those that exist already (oil and gas) is a practical point in their favour although the supplies will diminish since they are not renewable. The fuel distribution network has developed reasonably and is still growing and, we read that, the charging process (tank filling) made relatively easy. These factors have been bolstered by government financial incentives and other concessions so that adoption of the fuels has expanded to a significant level.We have little doubt that vested interests have played their part in the evolution that has taken place. Indeed, reading the sometimes misleading articles from the vendors, which flaunt the advantages without mentioning the core drawback, tends to confirm this suspicion.On balance we think it is better that vehicle operators are using these gases than sticking to the traditional petroleum fuels, but we believe that the government subsidies and concessions would have been better spent on alternatives such as biodiesel and organic alcohols.WORKS
Liquid Petroleum Gas (LPG)A Renewable FuelAlso known as LP-Gas, autogas and even simply propane, LPG is a mixture of mostly propane and butane gas that has been compressed into a liquid. It is the most commonly used alternative fuel in the world. The Gas Processors Association requires LPG to be at least 90% propane in order for it to qualify as a transportation fuel. Currently, about 2% of the energy used in the United States is from propane and less than 2% of that propane is used for transportation fuel. Properties Pressurized liquid, clear, colorless, non-toxic Production The chemicals that go into LPG, such as propane, are byproducts created during oil refining or through extracting heavy liquids during the processing of natural gas. They are separated from these products, then compressed into a liquid. CO2 Emissions YesLPG emits about half the carbon dioxide as conventional gasoline. Fuel Blends No Infrastructure In Place. Currently, the infrastructure for propane is the largest among all alternative fuels. In the US alone there are about 2,500 fueling stations in operation. Compared to Gasoline The statistics vary, but according to the Global Autogas industry Network (GAIN) , LPG emits only half as much carbon monoxide, 40% as much hydrocarbons and 35% as much nitrogen oxides as gasoline. Advantages • It has a high energy density and allows for good driving range.• If spilled, LPG presents no threat to soil, surface water, or groundwater because it evaporates immediately on meeting the surrounding air. Disadvantages • A gallon of propane has about 25% less energy than a gallon of gasoline.• Although supply currently exceeds demand, the supply itself is limited, meaning global conversion to autogas is unlikely• Converting a current vehicle to be able to run on LPG requires using the trunk space for the fuel tank. The weight of the tank also creates a subsequent drop in the car’s acceleration. The Future… … of autogas currently lies in at least two places. First, the development of the Liquid Propane Injection (LPI) engine will lead to substantially higher fuel efficiency, thereby making it all the more attractive to the consumer. Also, in 2007 Technology Review reported that researchers at MIT announced a chemical process for creating propane from sugarcane. The product, called biopropane, is a renewable fuel that could utilize propane’s existing infrastructure.
Tuesday, February 26, 2008
HYDROGEN FUEL CELL
A hydrogen vehicle is a vehicle that uses hydrogen as its on-board fuel for motive power. The term may refer to a personal transportation vehicle, such as an automobile, or any other vehicle that uses hydrogen in a similar fashion, such as an aircraft. The power plants of such vehicles convert the chemical energy of hydrogen to mechanical energy (torque) in one of two methods: combustion, or electrochemical conversion in a fuel-cell: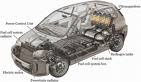
- In combustion, the hydrogen is burned in engines in fundamentally the same method as traditional gasoline cars.
- In fuel-cell conversion, the hydrogen is reacted with oxygen to produce water and electricity, the latter of which is used to power an electric traction motor.
The molecular hydrogen needed as an on-board fuel for hydrogen vehicles can be obtained through many thermochemical methods utilizing natural gas, coal (by a process known as coal gasification), liquefied petroleum gas, biomass (biomass gasification), by a process called thermolysis, or as a microbial waste product called biohydrogen or Biological hydrogen production. Hydrogen can also be produced from water by electrolysis. If the electricity used for the electrolysis is produced using renewable energy, the production of the hydrogen would (in principle) result in no net carbon dioxide emissions. On-board decomposition to produce hydrogen can occur when a catalyst is used.
Hydrogen is an energy carrier, not an energy source, so the energy the car uses would ultimately need to be provided by a conventional power plant. A suggested benefit of large-scale deployment of hydrogen vehicles is that it could lead to decreased emissions of greenhouse gases and ozone precursors.[1] Further, the conversion of fossil fuels would be moved from the vehicle, as in today's automobiles, to centralized power plants in which the byproducts of combustion or gasification can be better controlled than at the tailpipe. However, there are both technical and economic challenges to implementing wide-scale use of hydrogen vehicles, as well as better and less expensive alternatives. The timeframe in which challenges may be overcome is likely to be at least several decades, and hydrogen vehicles may never become broadly availableAdvantages of the hydrogen economy
In the previous section we saw the significant, worldwide problems created by fossil fuels. The hydrogen economy promises to eliminate all of the problems that the fossil fuel economy creates. Therefore, the advantages of the hydrogen economy include:- The elimination of pollution caused by fossil fuels - When hydrogen is used in a fuel cell to create power, it is a completely clean technology. The only byproduct is water. There are also no environmental dangers like oil spills to worry about with hydrogen.
- The elimination of greenhouse gases - If the hydrogen comes from the electrolysis of water, then hydrogen adds no greenhouse gases to the environment. There is a perfect cycle -- electrolysis produces hydrogen from water, and the hydrogen recombines with oxygen to create water and power in a fuel cell.
- The elimination of economic dependence - The elimination of oil means no dependence on the Middle East and its oil reserves.
- Distributed production - Hydrogen can be produced anywhere that you have electricity and water. People can even produce it in their homes with relatively simple technology.
As politicians and the public leap aboard the hydrogen fuel bandwagon, a University of California, Berkeley, energy expert suggests we all step back and take a critical look at the technology and consider simpler, cheaper options.
In a paper appearing in the July 18 issue of Science magazine, Alex Farrell, assistant professor of energy and resources at UC Berkeley, and David Keith, associate professor of engineering and public policy at Carnegie Mellon University, present various short- and long-term strategies that they say would achieve the same results as switching from gasoline-powered vehicles to hydrogen cars.
"Hydrogen cars are a poor short-term strategy, and it's not even clear that they are a good idea in the long term," said Farrell. "Because the prospects for hydrogen cars are so uncertain, we need to think carefully before we invest all this money and all this public effort in one area."
Farrell and Keith compared the costs of developing fuel cell vehicles to the costs of other strategies for achieving the same environmental and economic goals.
"There are three reasons you might think hydrogen would be a good thing to use as a transportation fuel - it can reduce air pollution, slow global climate change and reduce dependence on oil imports - but for each one there is something else you could do that would probably work better, work faster and be cheaper," Farrell said.
President George W. Bush has proposed a federally funded, five-year, $1.7 billion FreedomCAR and Fuel Initiative to develop hydrogen-powered fuel cells, a hydrogen infrastructure and advanced automotive technologies. Several announced candidates for president have also proposed major research efforts to develop hydrogen-fueled vehicles and technologies to produce, transport and store the hydrogen, while many scientists have praised the initiative.
For many people, the attraction of hydrogen is that it produces no pollution or greenhouse gases at the tailpipe. For others, the attraction is that hydrogen is a research program, not a regulation, and that some hydrogen-related research will also help develop better gasoline-powered cars.
One problem, said Farrell, an expert on energy and environment issues, is that this glosses over the issue of where the hydrogen comes from. Current methods of producing hydrogen from oil and coal produce substantial carbon dioxide. Unless and until this carbon can be captured and stored, renewable (wind or solar) and nuclear power, with their attendant problems of supply and waste, are the only means of producing hydrogen without also producing greenhouse gases.
In addition, Farrell points out that setting up a completely new infrastructure to distribute hydrogen would cost at least $5,000 per vehicle. Transporting, storing and distributing a gaseous fuel as opposed to a liquid raises many new problems.
More billions of dollars will be needed to develop hydrogen fuel cells that can match the performance of today's gasoline engines, he said.
The benefits might be worth the costs of fuel-cell development and creating a new infrastructure, however, if air pollution, greenhouse gases and imported petroleum could not be reduced in other ways. But they can, said Farrell.
Improvements to current cars and current environmental rules are more than 100 times cheaper than hydrogen cars at reducing air pollution. And for several decades, the most cost-effective method to reduce oil imports and CO2 emissions from cars will be to increase fuel efficiency, the two scientists found.
"You could get a significant reduction in petroleum consumption pretty inexpensively by raising the fuel economy standard or raising fuel prices, or both, which is probably the cheapest strategy," Farrell said. "This would actually have no net cost or possibly even a negative cost - buying less fuel would save more money than the price of the high-efficiency cars. The vehicles would still be large enough for Americans and they would still be safe."
Technologies are now on the shelf to achieve better fuel efficiency, he said. All that's lacking are economic incentives to encourage auto makers to make and drivers to buy fuel-efficient cars.
"Automobile manufacturers don't need to invest in anything fancy - a wide number of technologies are already on the shelf," he said, quoting, among other studies, a 2002 report by the National Academy of Sciences. "The cost would be trivial compared to the changes needed to go to a hydrogen car."
Petroleum substitutes like ethanol that can be used in today's vehicles also are a possible way to reduce oil imports, the researchers say, but more research is needed to reduce the environmental impact and cost of these options.
If one goal is to reduce greenhouse gases, it would be cheaper, Farrell and Keith argue, to focus on reducing carbon dioxide emissions from electric power plants than to focus solely on hydrogen-powered vehicles. But if passenger cars are targeted, fuel economy is still the key.
If it becomes necessary to introduce hydrogen into the transportation sector, the scientists say, a better alternative is to develop hydrogen-powered fuel cells for vehicles such as ships, trains and large trucks instead of cars. Because these heavy freight vehicles have higher emissions, this strategy could provide greater air quality benefits. On-board hydrogen storage would be less of a problem also, and it would require a smaller fuel distribution network.
Farrell and Keith provide figures that support their arguments and conclude that more research needs to be done before committing ourselves to a hydrogen economy, which might begin to make sense 25 years down the road.
"Hydrogen cars are an attractive vision that demands serious investigation, but it's not a sure thing," they wrote.
Farrell speculates that hydrogen has become attractive to people across the political spectrum in part because it doesn't challenge drivers to change their habits. It also doesn't challenge the auto industry to change its behavior, providing, instead, a subsidy for research that will lead to better cars whether they are hydrogen-powered or gasoline-powered.
Adapted from materials provided by University Of California - Berkeley.
EXAMPLE

DIESEL
Petroleum fuel, or crude oil, is naturally found in the Earth. When crude oil is refined at refineries, it can be separated into several different kinds of fuels, including gasoline, jet fuel, kerosene and, of course, diesel.
If you have ever compared diesel fuel and gasoline, you know that they are different. They certainly smell different. Diesel fuel is heavier and oilier. Diesel fuel evaporates much more slowly than gasoline -- its boiling point is actually higher than the boiling point of water. You will often hear diesel fuel referred to as "diesel oil" because it is so oily.
 Diesel fuel evaporates more slowly because it is heavier. It contains more carbon atoms in longer chains than gasoline does (gasoline is typically C9H20, while diesel fuel is typically C14H30). It takes less refining to create diesel fuel, which is why it used to be cheaper than gasoline. Since 2004, however, demand for diesel has risen for several reasons, including increased industrialization and construction in China and the U.S. [source: Energy Information Administration].
Diesel fuel evaporates more slowly because it is heavier. It contains more carbon atoms in longer chains than gasoline does (gasoline is typically C9H20, while diesel fuel is typically C14H30). It takes less refining to create diesel fuel, which is why it used to be cheaper than gasoline. Since 2004, however, demand for diesel has risen for several reasons, including increased industrialization and construction in China and the U.S. [source: Energy Information Administration].
Diesel fuel has a higher energy density than gasoline. On average, 1 gallon (3.8 L) of diesel fuel contains approximately 155x106 joules (147,000 BTU), while 1 gallon of gasoline contains 132x106 joules (125,000 BTU). This, combined with the improved efficiency of diesel engines, explains why diesel engines get better mileage than equivalent gasoline engines.
Diesel fuel is used to power a wide variety of vehicles and operations. It of course fuels the diesel trucks you see lumbering down the highway, but it also helps move boats, school buses, city buses, trains, cranes, farming equipment and various emergency response vehicles and power generators. Think about how important diesel is to the economy -- without its high efficiency, both the construction industry and farming businesses would suffer immensely from investments in fuels with low power and efficiency. About 94 percent of freight -- whether it's shipped in trucks, trains or boats -- relys on diesel.
In terms of the environment, diesel has some pros and cons. The pros -- diesel emits very small amounts of carbon monoxide, hydrocarbons and carbon dioxide, emissions that lead to global warming. The cons -- high amounts of nitrogen compounds and particulate matter (soot) are released from burning diesel fuel, which lead to acid rain, smog and poor health conditions. On the next page we'll look at some recent improvements made in these areas.Chemical composition
Petroleum-derived diesel is composed of about 75% saturated hydrocarbons (primarily paraffins including n, iso, and cycloparaffins), and 25% aromatic hydrocarbons (including naphthalenes and alkylbenzenes).[6] The average chemical formula for common diesel fuel is C12H23, ranging from approx. C10H20 to C15H28.
EXAMPLE
DIESEL CAR OF THE YEAR 2007
 merc DIESEL CAR OF THE YEAR 2005 jaguar
merc DIESEL CAR OF THE YEAR 2005 jaguar
GASOLINE-PETROL
Gasoline or petrol is a petroleum-derived liquid mixture consisting mostly of aliphatic hydrocarbons and enhanced with aromatic hydrocarbons toluene, benzene or iso-octane to increase octane ratings, primarily used as fuel in internal combustion engines. Most Commonwealth countries or former Commonwealth countries, with the exception of Canada, use the term "petrol" (abbreviated from petroleum spirit). The term "gasoline" is commonly used in North America where it is often shortened in colloquial usage to "gas". This should be distinguished in usage from genuinely gaseous fuels used in internal combustion engines such as liquified petroleum gas (which is stored pressurised as a liquid but is allowed to return naturally to a gaseous state before combustion). The term mogas, short for motor gasoline, distinguishes automobile fuel from aviation gasoline, or avgas. The word "gasoline" can also be used in British English to refer to a different petroleum derivative historically used in lamps; however, this use is now uncommon.
What is gasoline?
Gasoline is known as an aliphatic hydrocarbon. In other words, gasoline is made up of molecules composed of nothing but hydrogen and carbon arranged in chains. Gasoline molecules have from seven to 11 carbons in each chain. Here are some common configurations:H H H H H H H
H-C-C-C-C-C-C-C-H Heptane
H H H H H H H
H H H H H H H H
H-C-C-C-C-C-C-C-C-H Octane
H H H H H H H H
H H H H H H H H H
H-C-C-C-C-C-C-C-C-C-H Nonane
H H H H H H H H H
H H H H H H H H H H
H-C-C-C-C-C-C-C-C-C-C-H Decane
H H H H H H H H H H
When you burn gasoline under ideal conditions, with plenty of oxygen, you get carbon dioxide (from the carbon atoms in gasoline), water (from the hydrogen atoms) and lots of heat. A gallon of gasoline contains about 132x106 joules of energy, which is equivalent to 125,000 BTU or 36,650 watt-hours:
- If you took a 1,500-watt space heater and left it on full blast for a full 24-hour day, that's about how much heat is in a gallon of gas.
- If it were possible for human beings to digest gasoline, a gallon would contain about 31,000 food calories -- the energy in a gallon of gasoline is equivalent to the energy in about 110 McDonalds hamburgers!
CAR OF THE YEAR2007

HYBRID
- On-board rechargeable energy storage system (RESS) and
 a fueled power source (internal combustion engine or fuel cell)
a fueled power source (internal combustion engine or fuel cell) - Air and internal combustion engines
- Human powered bicycle with electric motor or gas engine assist
- Human-powered or sail boat with electric power
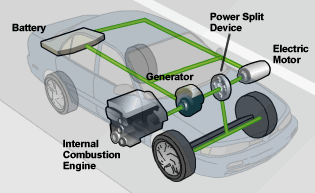
The auto industry has the technology to address these concerns. It's the hybrid car. There are a lot of hybrid models on the market these days, and most automobile manufacturers have announced plans to manufacture their own versions.
How does a hybrid automobile work? What goes on under the hood to give you 20 or 30 more miles per gallon than the standard automobile? And does it pollute less just because it gets better gas mileage? In this article, we'll help you understand how this technology works, and we'll even give you some tips on how to drive a hybrid car for maximum efficiency.
EXAMPLE
ELECTRIC
The electric car is a vehicle that utilizes chemical energy stored in rechargeable battery packs, and electric motors and motor controllers instead of internal combustion engines (ICEs).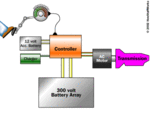
Vehicles using both electric motors and ICEs (hybrid electric vehicles) are examples of hybrid vehicles, and are not considered pure battery electric vehicles (BEVs) because they operate in a charge-sustaining mode. Hybrid vehicles with batteries that can be charged externally to displace some or all of their ICE power and gasoline fuel are called plug-in hybrid electric vehicles (PHEV), and are pure BEVs during their charge-depleting mode. BEVs include automobiles, light trucks, and neighborhood electric vehicles.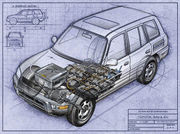
Electric cars were among the earliest automobiles. They produce no exhaust fumes, and minimal pollution if charged from most forms of renewable energy. Many are capable of acceleration exceeding that of conventional vehicles, are quiet, and do not produce noxious fumes. BEVs may reduce dependence on petroleum and decrease greenhouse gas emissions, depending on how their electricity is produced.
The Toyota RAV4 EV
was powered by twenty-four 12 volt batteries, with an operational cost equivalent of over 165 miles per gallon at 2005 US gasoline prices
Historically, BEVs and PHEVs have had issues with high battery costs, limited travel distance between battery recharging, charging time, and battery lifespan, which have limited widespread adoption. Ongoing battery technology advancements have addressed many of these problems; many models have recently been prototyped, and a handful of future production models have been announced. Toyota, Honda, Ford and General Motors all produced BEVs in the 90s in order to comply with the California Air Resources Board's Zero Emission Vehicle Mandate. The major US automobile manufacturers have been accused of deliberately sabotaging their electric vehicle production efforts.[1][2]
 Battery EVs may be cheaper to make and maintain than internal combustion engine vehicles because they have many fewer parts[citation needed]. Using regenerative braking, a feature which is standard on many electric and hybrid vehicles, a significant portion of energy may be recovered.[3][4]
Battery EVs may be cheaper to make and maintain than internal combustion engine vehicles because they have many fewer parts[citation needed]. Using regenerative braking, a feature which is standard on many electric and hybrid vehicles, a significant portion of energy may be recovered.[3][4]
In general terms a battery electric vehicle is a rechargeable electric vehicle. Other examples of rechargeable electric vehicles are ones that store electricity in ultracapacitors, or in a flywheel.
EXAMPLE
Monday, February 25, 2008
ETHANOL
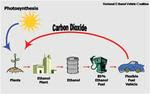
Ethanol also known as, ethyl alcohol, drinking alcohol or grain alcohol, is a flammable, colorless chemical compound, and is best known as the alcohol found in thermometers and alcoholic beverages. In common usage, it is often referred to simply as alcohol. It is a straight-chain alcohol and its molecular formula is variously represented as EtOH, CH3CH2OH, C2H5OH or as its empirical formula C2H6O (which it shares with dimethyl ether).
After the use of fire, fermentation of sugar into ethanol is perhaps the earliest organic reaction known to humanity, and the intoxicating effects of ethanol consumption have been known since ancient times. In modern times ethanol intended for industrial use has also been produced from byproducts of petroleum refining.
What is Bio Ethanol?
The principle fuel used as a petrol substitute for road transport vehicles is bio ethanol.
Ethanol or ethyl alcohol (C2H5OH) is a clear colourless liquid, it is biodegradable, low in toxicity and causes little environmental pollution if spilt. Ethanol burns to produce carbon dioxide and water. Ethanol is a high-octane fuel and has replaced lead as an octane enhancer in petrol. By blending ethanol with petrol we can also oxygenate the fuel mixture so it burns more completely and reduces polluting emissions. Ethanol fuel blends are widely sold in the United States and are becoming available here now. However, only flexible fuel vehicles or vehicles with one of our converters can run on 85% ethanol and 15% petrol blends (E85) which is the most common blend.
| Properties | |
|---|---|
| Molecular formula | C2H5OH |
| Molar mass | 46.06844(232) g/mol |
| Appearance | colorless clear liquid |
| Density | 0.789 g/cm³, liquid |
| Melting point | −114.3 °C (158.8 K) |
| Boiling point | 78.4 °C (351.6 K) |
| Solubility in water | Fully miscible |
| Acidity (pKa) | 15.9 (H+ from OH group) |
| Viscosity | 1.200 mPa·s (cP) at 20.0 °C |
| Dipole moment | 5.64 fC·fm (1.69 D) (gas) |
What is its History?
The use of ethanol in engines is not a new idea. In 1908, the Ford Model T was introduced and could run on ethanol or petrol. The difference in cost between the two fuels was what killed off the use of ethanol at that time.
But now, rising energy prices and environmental problems have led to increased interest in ethanol as a fuel. Ethanol has been used as a fuel in other points in history but fossil fuels have become the dominant energy resource for the modern world. Much attention has been placed on the prospects of using ethanol as fuel for cars.
How is it Produced?
Basic steps for dry mill production of ethanol are: refining into starch, liquification and saccharification (Starch to sugar), fermentation (Sugar to alcohol), distillation, dehydration, and denaturing (optional).
Many crops that are grown can be used for the process and include; corn, maize and wheat crops, waste straw, willow and popular trees, sawdust, reed canary grass, cord grasses, Jerusalem artichoke, myscanthus and sorghum plants.
EXAMPLE
FORD REFLEX HUMMER hx

BIO DEISEL

WHAT IS BIO DIESEL AND HOW IT WORKS
Biodiesel refers to a non-petroleum-based diesel fuel consisting of short chain alkyl (methyl or ethyl) esters, typically made by transesterification of vegetable oils or animal fats, which can be used (alone, or blended with conventional petrodiesel) in unmodified diesel-engine vehicles. Biodiesel is distinguished from the straight vegetable oil (SVO) (aka "waste vegetable oil", "WVO", "unwashed biodiesel", "pure plant oil", "PPO") used (alone, or blended) as fuels in some converted diesel vehicles. "Biodiesel" is standardized as methyl ester and other non-diesel fuels of biological origin are not included.[1]
Biodiesel is biodegradable and non-toxic, and typically produces about 60% less net-lifecycle carbon dioxide emissions, as it is itself produced from atmospheric carbon dioxide via photosynthesis in plants. Its emissions of smog forming hydrocarbon are 67% less, although the Nitrogen Oxide emissions are about 10% greater than those from petroleum-based diesel.[2][3] Net-lifetime carbon dioxide emissions can actually differ widely between fuels depending upon production methods of the source vegetable oils and processing methods employed in their creation. It is therefore debatable as to the extent that biodiesel reduces total carbon dioxide emissions currently contributing to anthropogenic global warming compared to those from petroleum-based diesel.
Biodiesel is safe and can be used in diesel engines with little or no modification needed. Although biodiesel can be used in its pure form, it is usually blended with standard diesel fuel. Blends are indicated by the abbreviation Bxx, where xx is the percentage of biodiesel in the mixture. For example, the most common blend is B20, or 20 percent biodiesel to 80 percent standard. So, B100 refers to pure biodiesel.
standard. So, B100 refers to pure biodiesel.
Biodiesel isn't just a catch-all term, however. There is also a formal, technical definition that is recognized by ASTM International (known formerly as the American Society for Testing and Materials), the organization responsible for providing industry standards. According to the National Biodiesel Board (NBB), the technical definition of biodiesel is as follows.
- a fuel comprised of mono-alkyl esters of long chain fatty acids derived from vegetable oils or animal fats, designated B100, and meeting the requirements of ASTM D 6751.
EXAMPLE
A BUS THAT RUNS ON BIO DIESEL





